Are you a US healthcare professional?
INDICATION
RYTELO® (imetelstat) is indicated for the treatment of adult patients with low- to intermediate-1 risk myelodysplastic syndromes (MDS) with transfusion-dependent anemia requiring 4 or more red blood cell units over 8 weeks who have not responded to or have lost response to or are ineligible for erythropoiesis-stimulating agents (ESA). See more
How to order
How RYTELO is supplied
RYTELO for injection is a white to off-white or slightly yellow powder supplied in a single-dose vial. Each carton contains one single-dose vial.1 Two vial sizes are available: 47 mg and 188 mg.
RYTELO coding


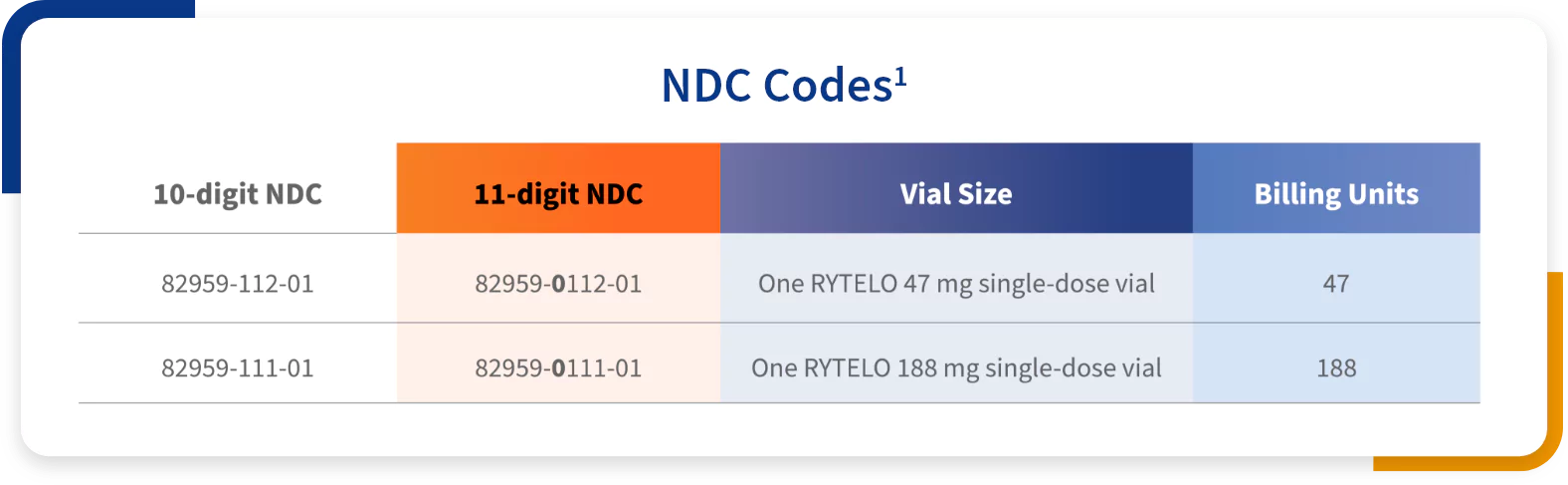
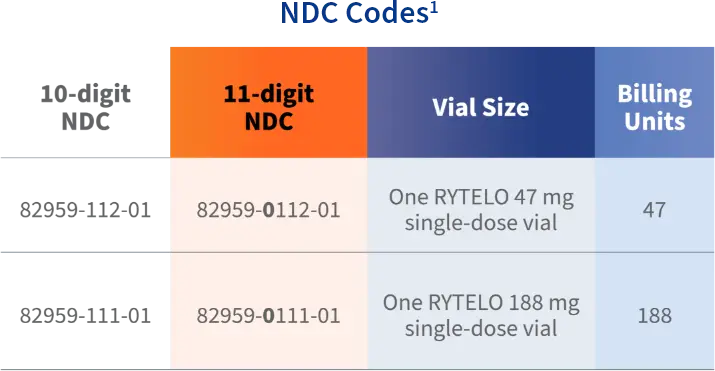
Guidelines for reporting the NDC number in the appropriate format, quantity, and unit of measure vary by state and by payer and should be reviewed prior to submitting a claim. It is important to identify and use the correct codes for each patient. Providers are responsible for all coding decisions. Geron does not guarantee coverage or reimbursement.
This information is provided for educational purposes only. It is always the provider’s responsibility to determine details specific to individual patients and to submit true and correct claims for the products and services rendered. Providers should contact third-party payers for specific information on their coding, coverage, payment policies, and fee schedules. Geron and its agents make no guarantee regarding reimbursement for any service or item. This resource is not intended as reimbursement advice, legal advice, medical advice, or a substitute for a provider’s independent professional judgment.
NDC, National Drug Code.
Reference: 1. RYTELO. Prescribing information. Geron Corp.; 2024.
INDICATION
RYTELO® (imetelstat) is indicated for the treatment of adult patients with low- to intermediate-1 risk myelodysplastic syndromes (MDS) with transfusion-dependent anemia requiring 4 or more red blood cell units over 8 weeks who have not responded to or have lost response to or are ineligible for erythropoiesis-stimulating agents (ESA).
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Thrombocytopenia
RYTELO® can cause thrombocytopenia based on laboratory values. In the clinical trial, new or worsening Grade 3 or 4 decreased platelets occurred in 65% of patients with MDS treated with RYTELO.
Monitor patients with thrombocytopenia for bleeding. Monitor complete blood cell counts prior to initiation of RYTELO, weekly for the first two cycles, prior to each cycle thereafter, and as clinically indicated. Administer platelet transfusions as appropriate. Delay the next cycle and resume at the same or reduced dose, or discontinue as recommended.
Neutropenia
RYTELO can cause neutropenia based on laboratory values. In the clinical trial, new or worsening Grade 3 or 4 decreased neutrophils occurred in 72% of patients with MDS treated with RYTELO.
Monitor patients with Grade 3 or 4 neutropenia for infections, including sepsis. Monitor complete blood cell counts prior to initiation of RYTELO, weekly for the first two cycles, prior to each cycle thereafter, and as clinically indicated. Administer growth factors and anti-infective therapies for treatment or prophylaxis as appropriate. Delay the next cycle and resume at the same or reduced dose, or discontinue as recommended.
Infusion-Related Reactions
RYTELO can cause infusion-related reactions. In the clinical trial, infusion-related reactions occurred in 8% of patients with MDS treated with RYTELO; Grade 3 or 4 infusion-related reactions occurred in 1.7%, including hypertensive crisis (0.8%). The most common infusion-related reaction was headache (4.2%). Infusion-related reactions usually occur during or shortly after the end of the infusion.
Premedicate patients at least 30 minutes prior to infusion with diphenhydramine and hydrocortisone as recommended and monitor patients for at least one hour following the infusion as recommended. Manage symptoms of infusion-related reactions with supportive care and infusion interruptions, decrease infusion rate, or permanently discontinue as recommended.
Embryo-Fetal Toxicity
Based on animal findings, RYTELO can cause embryo-fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with RYTELO and for 1 week after the last dose.
ADVERSE REACTIONS
Serious adverse reactions occurred in 32% of patients who received RYTELO. Serious adverse reactions in >2% of patients included sepsis (4.2%), fracture (3.4%), cardiac failure (2.5%), and hemorrhage (2.5%). Fatal adverse reactions occurred in 0.8% of patients who received RYTELO, including sepsis (0.8%).
Most common adverse reactions (≥10% with a difference between arms of >5% compared to placebo), including laboratory abnormalities, were decreased platelets, decreased white blood cells, decreased neutrophils, increased AST, increased alkaline phosphatase, increased ALT, fatigue, prolonged partial thromboplastin time, arthralgia/myalgia, COVID-19 infections, and headache.
Please see full Prescribing Information, including Medication Guide.
You are encouraged to report adverse events related to Geron products by calling 1-855-437-6664 (1-855-GERON-MI) (US only). If you prefer, you may contact the US Food and Drug Administration (FDA) directly. Visit www.fda.gov/MedWatch or call 1-800-FDA-1088.


